- Regulatory Status
- RUO
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

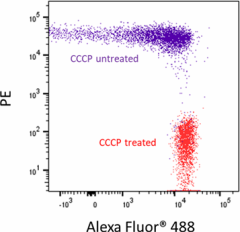
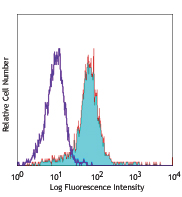
U937 cells treated (positive control, red) and untreated (negative control, purple) with CCCP then probed with the JC-10 Mitochondrial Membrane Potential Kit. The PE channel is used to detect JC-10 aggregates within mitochondria, while Alexa Fluor® 488 channel is used to detect JC-10 monomers. -
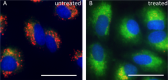
Live cell fluorescence imaging of HeLa cells without (panel A) and with (panel B) carbonyl cyanide m-chlorophenyl hydrazone (CCCP) treatment (5 µM for 30 min at 37°C), a potent mitochondrial decoupling agent. Then, cells were probed with JC-10 Mitochondrial Membrane Potential Kit. JC-10 aggregates can be visualized by red fluorescence, while JC-10 monomers which have diffused out of the mitochondria is visualized by green fluorescence. Nuclei were stained with Hoechst. Images were captured using a 40X objective. Scale bar: 15 µm -
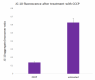
U937 cells were seeded onto a 96-well plate and left treated or untreated with CCCP for 30 minutes. The cells were then probed with the JC-10 Mitochondrial Membrane Potential Kit and analyzed on a fluorescence plate reader to determine the ratio of JC-10 aggregates (Em = 590 nm) to monomers (Em = 525 nm). A lower ratio indicates increased levels of mitochondrial membrane depolarization.
JC-10 is a cationic and lipophilic mitochondrial membrane potential probe which accumulates in the mitochondria of healthy cells. Accumulation of JC-10 aggregates in the mitochondrial matrix can be detected by its orange/red fluorescence (Ex/Em: 540/590 nm). During mitochondrial depolarization, a key marker of apoptosis, JC-10 diffuses out of the mitochondria and returns to its monomeric form which exhibits green fluorescence (Ex/Em: 490/525 nm). This behavior of JC-10 allows for ratiometric analysis of mitochondrial membrane potential, where a shift from orange to green fluorescence is indicative of compromised mitochondria. JC-10 can be used to qualitatively monitor and/or visualize mitochondrial membrane potential in live cells as a static endpoint or over time.
JC-10 was developed to offer an alternative to JC-1, which is widely used but has poor solubility in aqueous solutions. Issues with JC-1 solubility makes it difficult to utilize in some applications. The improved solubility of JC-10, however, allows for more versatility. This kit can be used for analysis of live cells by fluorescence microscopy, flow cytometry or plate reader analysis.
Kit Contents
- Kit Contents
-
- 100X JC-10 in DMSO (~3.5 mM) – 250 μL
- Dye Loading Buffer – 25 mL
- Masking Solution – 25 mL
Product Details
- Verified Reactivity
- Human, Mouse, Rat, All Species
- Formulation
- Each kit contains 1 vial of 100X JC-10 dye in DMSO (250 μL), 1 vial of Dye Loading Buffer (25 mL), 1 vial of Masking Solution (25 mL).
- Preparation
- Please refer to detailed protocol in application notes
- Storage & Handling
- -20°C
- Application
-
FC - Quality tested
Live cell imaging - Verified - Recommended Usage
-
Please refer to detailed protocol in application notes
- Application Notes
-
General Considerations:
- Optimal dye concentration and loading time will vary depending on cell type and application. Recommended dye concentrations range between 1-15 μM JC-10.
- JC-10 is compatible with kinetic imaging and analysis for up to 8 hrs after JC-10 addition under the appropriate experimental conditions.
- Carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone (FCCP) and carbonylcyanide-3-chlorophenylhydrazone (CCCP) are potent mitochondrial uncouplers which can be used for positive controls. An effective FCCP and CCCP concentration is typically 2-5 μM for 30 minutes at 37°C prior to dye addition for most cell types.
Reagent preparation:
- Allow all reagents to thaw and reach room temperature protected from light.
Note: Repeated freeze/thaw cycles of the JC-10 dye may cause loss of performance. We recommend storing the dye in one time use aliquots at -20°C. - Determine the desired amount of dye loading solution for your assay.
- Prepare the dye loading solution by diluting the 100X JC-10 stock solution 100-fold with the dye loading buffer. For example, add 10 μL JC-10 stock solution to 1 mL of dye loading buffer. Vortex until solution transitions from pink to near-colorless.
Flow Cytometry Assay:
- Harvest cells and resuspend in 100 μL of BioLegend Cell Staining Buffer (Cat. No. 420201), PBS, or HBSS at a density of 0.5 - 1x106 cells/tube.
- Add 50 μL of the prepared dye loading solution to each. This amount works well for most cell types.
- Incubate at room temperature or 37°C for 15-30 minutes.
Optional: Add up to 400 μL of additional buffer to adjust cell concentration before running on cytometer. - Analyze cells using a flow cytometer using the PE and FITC channels.
Note: For adjusting compensation, use FCCP/CCCP treated positive controls, which will have strong fluorescence in FITC channel with little or no signal in PE channel.
Note: Samples with healthy mitochondria will be positive in both channels but will have a stronger signal in the PE channel than FCCP/CCCP treated samples.
Microscopy Assay:
It is possible to load JC-10 dye prior to the addition of test compounds for real-time visualization of mitochondrial membrane depolarization. Time-lapse imaging of cells can continue for up to 8 hours after dye addition.- Seed cells into chamber slides or well plates with glass coverslip.
- Add dye loading solution directly to cells. Volume needed will vary depending on well size. We recommend a dilution of 1 part dye loading solution to 2 parts medium.
- Incubate cells for 30-60 min at 37°C protected from light.
- Add masking solution directly to cells. Volume needed will vary depending on well size. We recommend a dilution of 1 part masking solution to 3 parts total well volume. Do not remove dye loading solution from wells.
- Image cells using an inverted fluorescence microscope. JC-10 monomers can be visualized with standard FITC or GFP filters, and JC-10 aggregates can be viewed using TRITC or Texas Red® filters.
Note: Optimal imaging setup to simultaneously capture monomers and aggregates would be to use an excitation filter of ~490 nm with a long pass emission filter > 530 nm.
Plate Reader Assay:- Seed cells into a black 96-well plate and treat with test compounds of your choosing prior to the addition of dye loading solution. Final volume should be 100 μL/well.
- Add dye loading solution directly to cells. We recommend a dilution of 1 part dye loading solution to 2 parts medium.
- Incubate cells for 30-60 min at 37°C protected from light.
- Add masking solution directly to cells. Volume needed will vary depending on well size. We recommend a dilution of 1 part masking solution to 3 parts volume in well. Do not remove dye loading solution from wells.
- Measure fluorescence using a microplate reader for ratiometric analysis. To quantify JC-10 monomer fluorescence, use Ex/Em ~490/525 nm or instrument settings appropriate for fluorescein. To quantify JC-10 aggregate fluorescence, use Ex/Em ~540/590 nm or Texas Red® instrument settings.
-
Application References
(PubMed link indicates BioLegend citation) -
- Panusatid C, et al. 2022. MethodsX. 9:101685.
- Han L, et al. 2020. Medicine (Baltimore). 99:e22438.
- Li P, et al. 2022. Front Aging Neurosci. 14:869558.
- Yang M, et al. 2021. Front Immunol. 12:670088.
- Yokokura S, et al. 2014. BMC Cancer. 14:882.
Antigen Details
- Distribution
-
Mitochondrial matrix, cytoplasm
- Function
- Apoptosis, necrosis, mitochondrial membrane depolarization
- Gene ID
- NA














Follow Us