- Regulatory Status
- RUO
- Other Names
- INT2, INT-2, Heparin-binding growth factor 3, HNGF-3, HNGF3, FGF3
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-
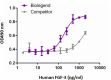
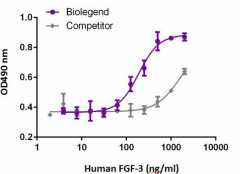
Human FGF‐3 induces NIH3T3 proliferation in the presence of 2 μg/mL of heparin, in a dose-dependent manner. BioLegend’s human FGF‐3 product was compared side‐by‐side to a competitor’s equivalent product.
FGF-3 is a member of the fibroblast growth factor family. The mouse FGF-3, originally named Int-2, was identified as a proto-oncogene that is activated by nearby integration of mouse mammary tumor viruses in virus-induced tumors. The amplification of the FGF-3 gene has been found in many cancer types including squamous cell carcinoma, epithelial ovarian tumors, breast cancer, and bladder cancer. FGF-3 is also associated with tumor metastasis and recurrence in human hepatocellular carcinoma. Like many other FGF proteins, FGF-3 plays important roles during embryonic development. FGF-3 is required for normal inner ear development including placode induction, maintenance, and otic vesicle formation. The role of FGF-3 in ear development is conserved amongst different vetebrates including mouse, chicken, and zebrafish. It has been shown that FGF-3, FGF-8, and FGF-10 play redundant and unique roles in ear development. FGF-3 is also important for early hindbrain patterning. The hindbrain boundary cells-derived FGF-3 regulates the expression of multiple markers at hindbrain boundaries. The involvement of FGF-3 in cardiovascular development also has been reported and is described to be in two forms. The two forms of FGF-3 describe a secreted form that induces proliferation and another form that localizes in the nucleus and inhibits cell proliferation. The nuclear isoform binds rpS2 and this binding has been suggested to interfere with ribosomal biogenesis.
Product DetailsProduct Details
- Source
- Human FGF-3, amino acids (Asp28-Arg212) (Accession# NP_005238), with an N-terminal Met and a 6-His tag was expressed in E.coli.
- Molecular Mass
- The 192 amino acid recombinant protein has a predicted molecular mass of approximately 22 kD. The DTT-reduced and non-reduced protein migrate at approximately 22 kD by SDS-PAGE. The predicted N-terminal amino acid is Met.
- Purity
- >95%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 20 mM Tris, pH 7.0, 100 mM NaCl, 1 mM EDTA, and 10% glycerol.
- Endotoxin Level
- Less than 0.01ng per µg cytokine as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- The ED50 is 100 - 200 ng/ml, corresponding to a specific activity of 5.0 -10 x 103 units/mg, as determined by a dose-dependent stimulation of NIH3T3 cell proliferation in the presence of 2 µg/ml of heparin.
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Growth factor.
- Distribution
-
Hindbrain, hindbrain boundary cells, the pharyngeal endoderm, and cancer cells.
- Function
- FGF-3 plays important roles in embryonic development, cell proliferation, and differentiation. Retinoic acid regulates the signal transduction of FGF-3 in the inner ear development
- Interaction
- Ribosomal protein S2
- Ligand/Receptor
- FGF-3 binds to the IIIb isoforms of FGFR1 and FGFR2 with high affinity and binds to the IIIc isoforms of FGFR2 with low affinity.
- Cell Type
- Embryonic Stem Cells, Hematopoietic stem and progenitors, Mesenchymal Stem Cells, Neural Stem Cells
- Biology Area
- Cell Biology, Neuroscience, Stem Cells, Synaptic Biology
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Kiefer P, et al. 1991. Mol. Cell Biol. 11:5929.
2. Brookes S, et al. 1989. Oncogene 4:429.
3. Zelarayan LC, et al. 2007. Dev. Biol. 308:379.
4. Fekete DM. 2000. Trends Neurosci. 23:332.
5. Leger S and Brand M. 2002. Mech. Dev. 119:91.
6. Weisinger K, et al. 2012. Biol. Open. 1:67.
7. Hatch EP, et al. 2007. Development 134:3615.
8. Antoine M, et al. 2005. Biochem. Biophys. Res. Commun. 338:1248.
9. Frenz DA, et al. 2010. Am. J. Med. Genet. A. 152A:2947. - Gene ID
- 2248 View all products for this Gene ID
- UniProt
- View information about FGF-3 on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)









Follow Us