- Regulatory Status
- RUO
- Other Names
- SLIL3, Slit homolog 2, Slit Guidance Ligand
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

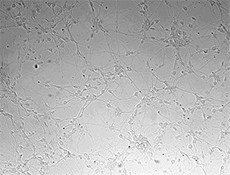
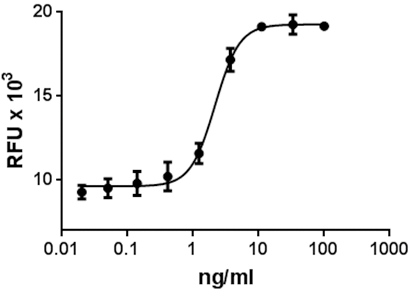
Immobilized human Slit2-N at 2.5 µg/mL enhances neurite outgrowth from E18 rat embryonic cortical neurons cultured for 3 days. -
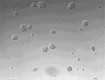
Cortical neurons cultured on PBS coated well for 3 days.
| Cat # | Size | Price | Save |
|---|---|---|---|
| 757104 | 25 µg | ¥44,380 |
This product is not available for shipping outside of the United States.
Slit glycoproteins and their Roundabout (ROBO) receptors were originally identified as important axon guidance molecules during the neural development. Slit1 and Slit2 are essential for midline guidance in the forebrain by acting as repulsive signal to axons projecting from the olfactory bulb. In the spinal cord, Slit proteins guide commissural axons by modulating their response to netrin at the floor plate where only commissural axons that crossed the midline respond to Slit2. Slit2 also play a role in branching and arborization of CNS sensory axons, in neuronal cell migration, and in regulating leukocyte migration. Slit2 is expressed primarily in the fetal lung, kidney, and adult spinal cord, and to a lesser extent in adult adrenal gland, thyroid, and trachea. Slit2 is initially synthesized as a 1499 amino acid precursor, which is subsequently cleaved into N-terminal and C-terminal fragments, designated as Slit2-N and Slit2-C respectively. The neurodevelopment related activities are contained only in the N-terminal fragment. Slit2 binds to multiple receptors including ROBO-1, -2, -3, Laminin-1, DAN, Gremlin, and Glypican-1. Slit2 has been shown to promote angiogenesis via ROBO-1 and ROBO-2 in a mouse model of ocular neovascular disease.
Product DetailsProduct Details
- Source
- Human Slit2-N, amino acids Gln26-Val1118 (Accession# O94813) was expressed in HEK293.
- Molecular Mass
- The 1093 amino acid recombinant protein has a predicted molecular mass of approximately 122.3 kD. The protein migrates at 120.0-140.0 kD under reducing condition by SDS-PAGE. The predicted N-terminal amino acid is Gln.
- Purity
- >98%, as determined by SDS-PAGE gel and HPLC analysis.
- Formulation
- Lyophilized from 0.2 µm filtered protein solution with no additives.
- Endotoxin Level
- Less than 1 EU per µg protein as determined by the LAL method.
- Storage & Handling
- Unopened vial can be stored at -20°C or -70°C. For maximum results, quick spin vial prior to opening. Reconstitute in 20 mM Tris, 150 mM NaCl, pH 8.8 to a concentration of 0.1-0.5 mg/ml. Do not vortex. For extended storage, it is recommended to further dilute in a buffer containing a carrier protein such as 0.1% BSA and store working aliquots at -20°C to -80°C. Avoid repeated freeze/thaw cycles.
- Activity
- Immobilized protein enhances neurite outgrowth at 2.5 - 10 µg/mL.
- Application
-
Bioassay
Antigen Details
- Distribution
-
Fetal lung and kidney, and adult spinal cord. Weak expression in adult adrenal gland, thyroid, trachea.
- Function
- Axon guidance, branching, arborization, neuronal and leukocyte migration.
- Interaction
- Commissural axon, Olfactory bulb axon, CNS sensory neurons, and leukocyte.
- Ligand/Receptor
- ROBO-1, ROBO-2, ROBO-3, Laminin-1, DAN, Gremlin, Glypican-1.
- Bioactivity
- Measured by the ability of immobilized human Slit2-N to enhance neurite outgrowth from E18 rat cortical neurons.
- Biology Area
- Cell Biology, Neuroscience, Synaptic Biology
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Brose K, et al. 1999. Cell 96:795-806.
2. Kidd T, et al. 1999. Cell 96:785-794.
3. Wu W, et al. 1999. Nature 400:331-336.
4. Stein E, et al. 2001. Science 291:1928-1938.
5. Bagri A, et al. 2002. Neuron 33:233-248.
6. Plump AS, et al. 2002. Neuron 33:219-232.
7. Grieshammer U, et al. 2004. Dev. Cell 6:709-717.
8. Zhou WJ, et al. 2013. Nature 501:107-111.
9. Rama N, et al. 2015. Nat. Med. 21:483-491. - Gene ID
- 9353 View all products for this Gene ID
- UniProt
- View information about Slit2-N on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)









Follow Us