- Regulatory Status
- RUO
- Other Names
- Coronavirus spike Protein RBD, S1 RBD, Spike Protein RBD, Mink variant
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

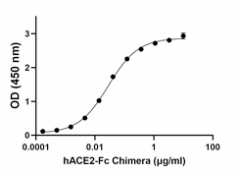
When recombinant SARS-CoV-2 Mink Variant RBD is immobilized at 2 μg/mL, recombinant human ACE2-Fc Chimera (Cat. No. 793202) binds in a dose-dependent manner. The ED50 for this effect is 10 – 50 ng/mL. HRP Protein A (Cat. No. 689202) was used to detect the binding. -

Stability Testing for Recombinant SARS-CoV-2 Mink Variant RBD. Recombinant SARS-CoV-2 Mink Variant RBD was aliquoted in PBS at 0.2 mg/mL. One aliquot was frozen and thawed four times (4x Freeze/Thaw), and compared to a control kept at 4⁰C (Control). The samples were tested by their ability to bind recombinant human ACE2-Fc chimera in a dose dependent-manner. The ED50 for this effect is 10 – 50 ng/mL.
| Cat # | Size | Price | Save |
|---|---|---|---|
| 797104 | 25 µg | ¥81,790 | |
| 797106 | 100 µg | ¥185,470 |
SARS-CoV-2 is a respiratory virus which causes coronavirus disease 2019 (COVID-19). The coronavirus spike (S) glycoprotein is a class I viral fusion protein on the outer envelope of the virion that plays a critical role in viral infection by recognizing host cell receptors and mediating fusion of the viral and cellular membranes. The S glycoprotein is synthesized as a precursor protein consisting of ~1,300 amino acids that is then cleaved into an amino (N)-terminal S1 subunit (~700 amino acids) and a carboxyl (C)-terminal S2 subunit (~600 amino acids). Three S1/S2 heterodimers assemble to form a trimer spike protruding from the viral envelope. The S1 subunit contains a receptor-binding domain (RBD) that can specifically bind to angiotensin-converting enzyme 2 (ACE2), the receptor on target cells. Triggered by receptor binding, proteolytic processing, and/or acidic pH in the cellular compartments, the class I viral fusion protein undergoes a transition from a metastable pre-fusion state to a stable post-fusion state during infection. During this, the receptor-binding subunit is cleaved, and the fusion subunit undergoes large-scale conformational rearrangements to expose the hydrophobic fusion peptide, inducing the formation of a six-helix bundle, bringing the viral and cellular membranes close for fusion. The trimeric SARS coronavirus (SARS-CoV-2) S glycoprotein consisting of three S1-S2 heterodimers binds the cellular receptor angiotensin-converting enzyme 2 (ACE2) and mediates fusion of the viral and cellular membranes through a pre- to post-fusion conformation transition. SARS-CoV-2 Mink variant was initially identified by mink farmers in Denmark. Mink variant has the mutations Y453F/D614G and presents an increased affinity for human ACE2. The SARS-CoV-2 Mink variant represents a model of sarbecovirus interspecies evolution.
Product DetailsProduct Details
- Source
- SARS-CoV-2 Mink Variant RBD amino acid Arg319-Phe541 (Accession # QHD43416.1), with a C-terminal 8His tag was expressed in CHO cells.
- Molecular Mass
- The 234 amino acid recombinant protein has a predicted molecular mass of approximately 26.3 kD. The DTT-reduced and non-reduced proteins migrate at approximately 35 kD and 30 kD by SDS-PAGE, respectively. The predicted N-terminal amino acid is Arg.
- Purity
- > 95%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in PBS, pH 7.2.
- Endotoxin Level
- Less than 0.1 EU per µg protein as determined by the LAL method.
- Concentration
- 25 µg size is bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- When recombinant SARS-CoV-2 Mink Variant RBD is immobilized at 2 μg/mL, recombinant human ACE2-Fc Chimera (Cat. No. 793202) binds in a dose-dependent manner. The ED50 for this effect is 10 – 50 ng/mL. HRP Protein A (Cat. No. 689202) was used to detect the binding.
- Application
-
Bioassay
- Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are validated in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Monomer
- Distribution
-
SARS-CoV-2
- Function
- Attaches the virion to the cell membrane by interacting with host receptor (ACE2), initiating the infection
- Interaction
- Ciliated cells in nasal mucosa and bronchus, pneumocytes
- Ligand/Receptor
- ACE2
- Bioactivity
- Measured by its ability to bind to recombinant human ACE2-Fc chimera
- Cell Type
- B cells, Endothelial cells, Macrophages, Monocytes, NK cells, T cells, Th1
- Biology Area
- Adaptive Immunity, Immunology, Innate Immunity
- Antigen References
-
- Lu R, et al. 2020. Lancet. 395:565-574.
- Li F. 2016. Annu Rev Virol. 3:237-61.
- Belouzard S, et al. 2012. Viruses. 4(6):1011-33.
- Song W, et al. 2018. PLoS Pathog. 14:e1007236
- Li F, et al. 2020. Nature. 581:221-4.
- Gene ID
- 43740568 View all products for this Gene ID
- UniProt
- View information about Spike RBD on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)








Follow Us