Resting vs. Activated Tregs
Similar to other T cells, Tregs originate from the thymus. However, Tregs exhibit distinct phenotypes and functions based on their activation status. These subpopulations1 include:
Resting Tregs (rTregs) are characterized by CD45RA+ FOXP3low expression. rTregs are primarily quiescent and play a crucial role in immune homeostasis. They are suppressive in vitro, and maintain immune tolerance and prevent autoimmunity. Activated Tregs (aTregs) are identified by CD45RA- FOXP3high expression. They are also suppressive in vitro, but more dynamic and responsive. aTregs engage in cytokine secretion and actively regulate immune responses.
Naturally occurring Tregs (nTregs) are characterized by the expression of CD4, CD25 and FOXP3. In addition to nTregs, there are several distinct subsets of induced regulatory T cells (iTregs), including Tr1 and Th3. Both Tr1 and Th3 cells contribute to immune regulation by producing and secreting IL-10 and TGF-β to dampen immune responses. These cells often do not fully replicate the functional or phenotypic characteristics of in vivo generated peripheral Tregs (pTregs) and questions remain regarding: the T cell receptor (TCR) repertoire and function of pTregs; the conditions under which they are generated in vivo; and the extent to which these characteristics distinguish pTregs from thymus Tregs (tTregs). Recent advances, including the discovery of unique cell surface markers and transcription factors (such as Neuropilin-1 and Helios), have shed light on the biology of tTregs versus pTregs.
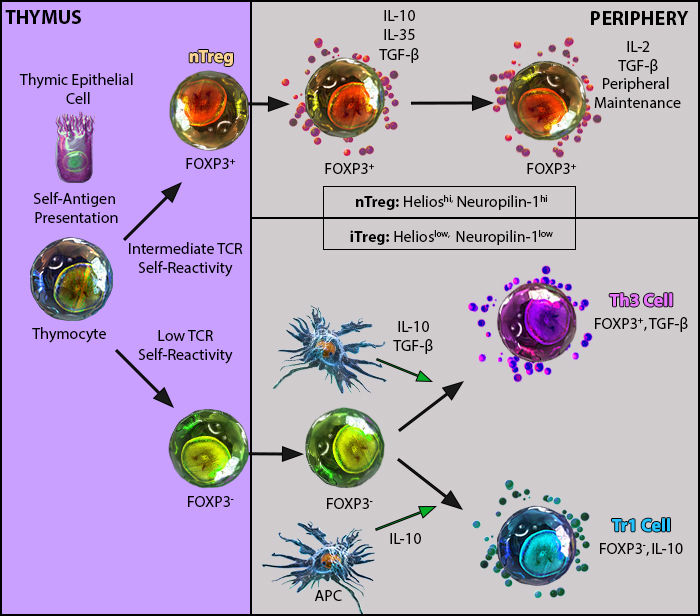
nTregs arise in the thymus while iTregs can develop in the periphery1.
Tissue Resident Tregs
While Tregs are well-known for their effects in immune tissues, evidence suggests that they also significantly impact non-lymphoid peripheral tissues as resident cells, such as in the skin and muscle. These tissue-resident Tregs, also known as tissue Tregs, do not recirculate in the blood or lymphatics. They exhibit distinct transcriptional and immune cell phenotypes compared to common Tregs in circulation.
Tregs limit immune activation through a variety of direct and indirect interactions. Thus, understanding tissue Tregs could guide clinical treatments, especially in diseases affecting local tissue homeostasis. Deciphering the balance between Treg subsets is essential for deciphering their roles in health, disease, and therapeutic interventions.
Treg Suppression Mechanisms
Tregs orchestrate immune tolerance through a combination of cytokines, direct cell interactions, and metabolic regulation. Tregs produce and release immunosuppressive cytokines. These include TGF-β that suppresses immune responses by inhibiting T cell activation and proliferation, as well as IL-10 to downregulate inflammation, and IL-35 to hamper effector T cell responses.
Tregs can also directly kill effector T cells by producing molecules such as granzyme and perforin which helps maintain immune tolerance. Tregs can modulate dendritic cell function and maturation by influencing antigen presentation. They also inhibit the proliferative response of effector T cells through several mechanisms, such as IL-2 receptor inhibition and limiting IL-2 availability, which is essential for T cell proliferation. Additionally, Tregs can also elevate intracellular cyclic AMP levels, suppressing T cell activation2.
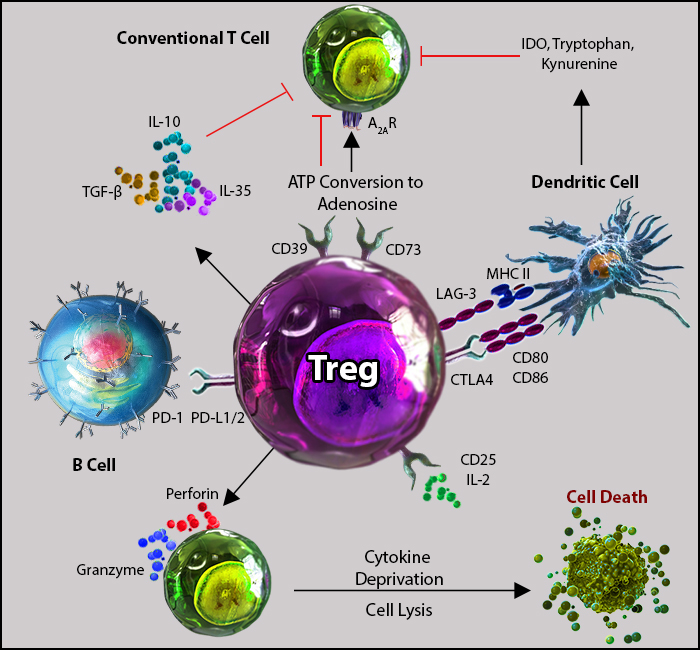
Tregs possess many mechanisms of action to induce immunosuppression2.
References
1. Workman, Creg J et al. “The development and function of regulatory T cells.” Cellular and molecular life sciences: CMLS vol. 66,16 (2009): 2603-22. doi:10.1007/s00018-009-0026-2
2. Xu, Zhan et al. “The Dynamic Role of FOXP3+ Tregs and Their Potential Therapeutic Applications During SARS-CoV-2 Infection.” Frontiers in immunology vol. 13 916411. 8 Jul. 2022, doi:10.3389/fimmu.2022.916411
 Login / Register
Login / Register 






Follow Us