- Clone
- OKT4 (See other available formats)
- Regulatory Status
- RUO
- Workshop
- HCDM listed
- Other Names
- T4
- Isotype
- Mouse IgG2b, κ
- Ave. Rating
- Submit a Review
- Product Citations
- publications
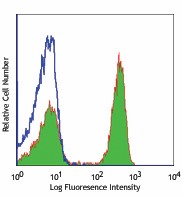
-

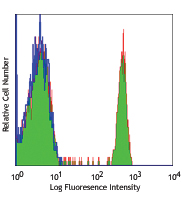
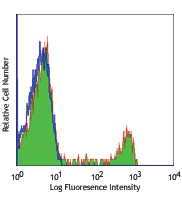
Human peripheral blood lymphocytes stained with Ultra-LEAF™ purified OKT4, followed by anti-mouse IgGs FITC
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 317453 | 100 µg | 190€ | ||||
| 317454 | 1 mg | 542€ | ||||
CD4, also known as T4, is a 55 kD single-chain type I transmembrane glycoprotein expressed on most thymocytes, a subset of T cells, and monocytes/macrophages. CD4, a member of the Ig superfamily, recognizes antigens associated with MHC class II molecules and participates in cell-cell interactions, thymic differentiation, and signal transduction. CD4 acts as a primary receptor for HIV, binding to HIV gp120. CD4 has also been shown to interact with IL-16.
Product DetailsProduct Details
- Verified Reactivity
- Human, Cynomolgus, Rhesus
- Reported Reactivity
- Chimpanzee
- Antibody Type
- Monoclonal
- Host Species
- Mouse
- Immunogen
- Human peripheral T cells
- Formulation
- 0.2 µm filtered in phosphate-buffered solution, pH 7.2, containing no preservative.
- Endotoxin Level
- Less than 0.01 EU/µg of the protein (< 0.001 ng/µg of the protein) as determined by the LAL test.
- Preparation
- The Ultra-LEAF™ (Low Endotoxin, Azide-Free) antibody was purified by affinity chromatography.
- Concentration
- The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C. This Ultra-LEAF™ solution contains no preservative; handle under aseptic conditions.
- Application
-
FC - Quality tested
IHC-F - Reported in the literature, not verified in house - Recommended Usage
-
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 2.0 µg per million cells in 100 µL volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Application Notes
-
The OKT4 antibody binds to the D3 domain of CD4 and does not block HIV binding. Additional reported applications (for the relevant formats) include: immunohistochemistry of frozen sections and blocking of T cell activation. This clone was tested in-house and does not work on formalin fixed paraffin-embedded (FFPE) tissue. The Ultra-LEAF™ purified antibody (Endotoxin < 0.01 EU/µg, Azide-Free, 0.2 µm filtered) is recommended for functional assays (Cat. No. 317453 and 317454).
In a small subset of individuals, the OKT4 clone does not bind to CD4 due to polymorphisms in CD4.9 -
Application References
(PubMed link indicates BioLegend citation) -
- Knapp W, et al. 1989. Leucocyte Typing IV. Oxford University Press. New York.
- Reinherz EL, et al. 1979. Proc. Natl. Acad. Sci. 76:4061.
- Kmieciak M, et al. 2009. J. Transl. Med. 7:89. (FC) PubMed
- Cicin-Sain L, et al. 2010. J. Immunol. 184:6739. PubMed
- Rosenzweig M, et al. 2001. J. Med. Primatol. 30:36.
- Linder J, et al. 1987. Am. J. Pathol. 127:1.
- Boche D, et al. 1999. J. Neurovirol. 5:232. (IHC)
- Reinherz EL, et al. 1979. Proc. Natl. Acad. Sci. USA. 76:4061. (Immunogen)
- Lederman S, et al. 1991. Mol Immunol. 28:1171-81.
- RRID
-
AB_2876620 (BioLegend Cat. No. 317453)
AB_2876620 (BioLegend Cat. No. 317454)
Antigen Details
- Structure
- Ig superfamily, type I transmembrane glycoprotein, 55 kD
- Distribution
-
T cell subset, majority of thymocytes, monocytes/macrophages
- Function
- MHC class II co-receptor, lymphocyte adhesion, thymic differentiation, HIV receptor
- Ligand/Receptor
- MHC class II molecules, HIV gp120, IL-16
- Cell Type
- Macrophages, Monocytes, T cells, Thymocytes, Tregs
- Biology Area
- Immunology
- Molecular Family
- CD Molecules
- Antigen References
-
1. Center D, et al. 1996. Immunol. Today 17:476.
2. Gaubin M, et al. 1996. Eur. J. Clin. Chem. Clin. Biochem. 34:723. - Gene ID
- 920 View all products for this Gene ID
- UniProt
- View information about CD4 on UniProt.org
Related FAQs
- I am unable to see expression of T cell markers such as CD3 and CD4 post activation.
- TCR-CD3 complexes on the T-lymphocyte surface are rapidly downregulated upon activation with peptide-MHC complex, superantigen or cross-linking with anti-TCR or anti-CD3 antibodies. PMA/Ionomycin treatment has been shown to downregulate surface CD4 expression. Receptor downregulation is a common biological phenomenon and so make sure that your stimulation treatment is not causing it in your sample type.
- Do you guarantee that your antibodies are totally pathogen free?
-
BioLegend does not test for pathogens in-house aside from the GoInVivo™ product line. However, upon request, this can be tested on a custom basis with an outside, independent laboratory.
- Does BioLegend test each Ultra-LEAF™ antibody by functional assay?
-
No, BioLegend does not test Ultra-LEAF™ antibodies by functional assays unless otherwise indicated. Due to the possible complexities and variations of uses of biofunctional antibodies in different assays and because of the large product portfolio, BioLegend does not currently perform functional assays as a routine QC for the antibodies. However, we do provide references in which the antibodies were used for functional assays and we do perform QC to verify the specificity and quality of the antibody based on our strict specification criteria.
- Does BioLegend test each Ultra-LEAF™ antibody for potential pathogens?
-
No, BioLegend does not test for pathogens in-house unless otherwise indicated. However, we can recommend an outside vendor to perform this testing as needed.
- Have you tested this Ultra-LEAF™ antibody for in vivo or in vitro applications?
-
We don't test our antibodies for in vivo or in vitro applications unless otherwise indicated. Depending on the product, the TDS may describe literature supporting usage of a particular product for bioassay. It may be best to further consult the literature to find clone specific information.
Other Formats
View All CD4 Reagents Request Custom ConjugationCustomers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Brilliant Violet 650™ anti-human CD4
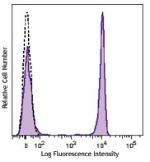
Human peripheral blood lymphocytes were stained with CD4 (cl... -
Purified anti-human CD4
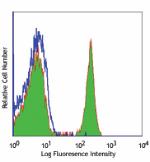
Human peripheral blood lymphocytes stained with purified OKT... 
Human frozen spleen tissue slices were fixed with 4% PFA for... -
Biotin anti-human CD4
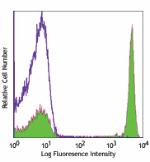
Human peripheral blood lymphocytes stained with biotinylated... -
FITC anti-human CD4
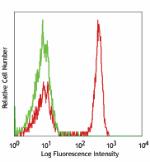
Human peripheral blood lymphocytes stained with OKT4 FITC -
PE anti-human CD4
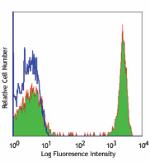
Human peripheral blood lymphocytes stained with OKT4 PE 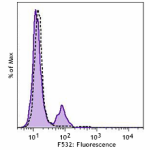
Human peripheral blood was stained with CD4 (clone OKT4) PE ... -
PE/Cyanine5 anti-human CD4
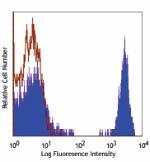
Human peripheral blood lymphocytes stained with OKT4 PE/Cyan... -
PE/Cyanine7 anti-human CD4
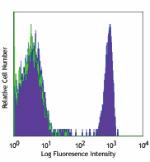
Human peripheral blood lymphocytes stained with OKT4 PE/Cyan... -
APC anti-human CD4
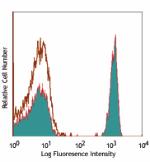
Human peripheral blood lymphocytes stained with OKT4 APC -
APC/Cyanine7 anti-human CD4
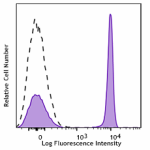
Human peripheral blood lymphocytes stained with CD4 (clone O... -
Alexa Fluor® 488 anti-human CD4
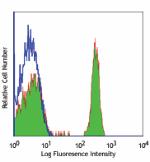
Human peripheral blood lymphocytes stained with OKT4 Alexa F... 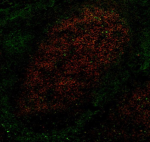
Human frozen tonsil tissue slices were fixed with 4% PFA for... -
Alexa Fluor® 647 anti-human CD4
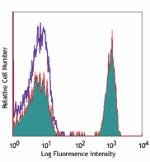
Human peripheral blood lymphocytes stained with OKT4 Alexa F... 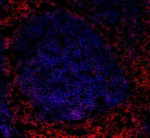
Human frozen tonsil tissue slices were fixed with 4% PFA for... -
Alexa Fluor® 700 anti-human CD4
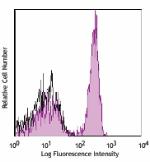
Human peripheral blood lymphocytes stained with OKT4 Alexa F... -
Pacific Blue™ anti-human CD4
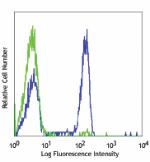
Human peripheral blood lymphocytes stained with OKT4 Pacific... -
PerCP/Cyanine5.5 anti-human CD4
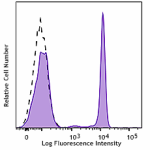
Human peripheral blood lymphocytes were stained with CD4 (cl... -
PerCP anti-human CD4
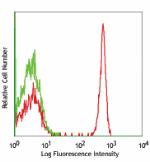
Human peripheral blood lymphocytes stained with OKT4 PerCP -
Brilliant Violet 421™ anti-human CD4
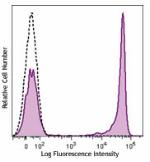
Human peripheral blood lymphocytes were stained with CD4 (cl... 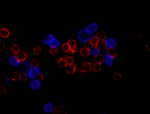
Human peripheral mononuclear cells were fixed with 2% parafo... -
Brilliant Violet 605™ anti-human CD4
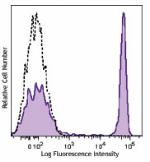
Human peripheral lymphocytes were stained with CD4 (clone OK... -
Brilliant Violet 711™ anti-human CD4
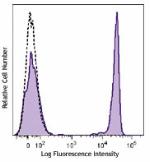
Human peripheral blood lymphocytes were stained with CD4 (cl... -
Brilliant Violet 785™ anti-human CD4
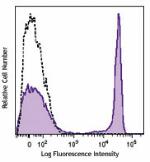
Human peripheral lymphocytes were stained with CD4 (clone OK... -
Brilliant Violet 510™ anti-human CD4
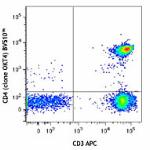
Human peripheral lymphocytes were stained with CD3 APC and C... -
Brilliant Violet 570™ anti-human CD4
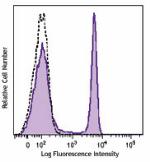
Human peripheral lymphocytes were stained with CD4 (clone OK... -
PE/Dazzle™ 594 anti-human CD4
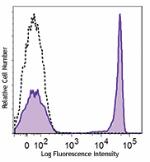
Human peripheral blood lymphocytes were stained with CD4 (cl... -
TotalSeq™-A0922 anti-human CD4
-
Ultra-LEAF™ Purified anti-human CD4
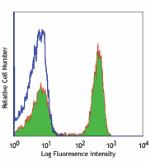
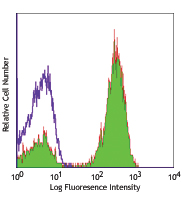
Human peripheral blood lymphocytes stained with Ultra-LEAF™ ... -
TotalSeq™-C0922 anti-human CD4
-
TotalSeq™-B0922 anti-human CD4
-
PE/Fire™ 810 anti-human CD4
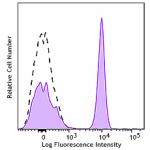
Human peripheral blood lymphocytes were stained with anti-hu... -
APC/Fire™ 750 anti-human CD4 Antibody

Human peripheral blood lymphocytes were stained with anti-hu...
 Login / Register
Login / Register 









Follow Us