BioLegend Cell-Vive™ GMP recombinant proteins are manufactured and tested in accordance with USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 in a dedicated ISO 13485:2016 certified GMP facility.
Find complete GMP manufacturing quality and regulatory statements here.
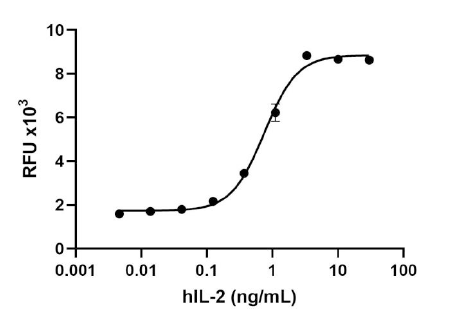
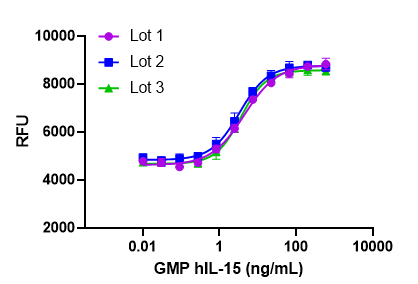
Our GMP recombinant proteins are ideal for use in expansion, polarization, and differentiation of cells for research and ex vivo cell processing applications. BioLegend GMP recombinant proteins are also free of BSA or other equivalent carrier proteins, thus eliminating the risk of introducing nonrelated or undesired effects on experiments.
 Login/Register
Login/Register 








Follow Us