B Cells and T Cells
B cells and T cells are the major cell types involved in adaptive immunity and must be able to recognize and respond to millions of possible antigens in order to function effectively. To achieve this, they create almost infinite diversity at their cell surface receptors, which comprise antibodies in the case of B cells and antibody-like structures known as T cell receptors (TCRs) at the surface of T cells.
Antibody diversity at the B cell surface is driven by three main mechanisms. The first of these is V(D)J recombination, which occurs during early B cell development and involves the splicing of different variable (V), diversity (D), and joining (J) gene segments to form the antibody variable region. Because so many different V, D, and J gene segments are available, and because they are combined in such a random fashion, millions of genetic configurations are possible. Junctional diversification provides further variation at this stage through the removal or addition of nucleotides at the ends of the V-region coding sequences as they are joined by the V(D)J recombinase enzyme complex. Thirdly, when a mature B cell encounters the antigen recognized by the B cell receptor, its recombined genes can undergo a third level of diversification known as somatic hypermutation. Here, an enzyme called activation-induced cytidine deaminase (AID) introduces random point mutations into the antibody variable region genes to further diversify the antibody population. Somatic hypermutation results in the development of higher affinity antibodies as the adaptive immune response progresses, a process referred to as affinity maturation.
When a B cell meets its antigen, it can undergo one of two fates. Differentiation into IgM-secreting plasma cells provides a rapid yet short-lived response to the target, with pentameric IgM antibodies being involved in neutralization and clearance, as well as initiating inflammatory reactions via the complement system. Alternatively, B cells can proliferate and undergo affinity maturation, producing large quantities of IgG antibodies and additionally developing into memory B cells able to respond efficiently should the antigen be encountered again.
Rather than being comprised of an antibody, the T cell receptor (TCR) consists of a disulfide-linked heterodimer of alpha (α) and beta (β) chains that form a complex with CD3 at the T cell surface (although it should be noted a much smaller fraction of T cells have a TCR consisting of a gamma chain and a delta chain). Each of the α and β chains includes a constant region and a variable region, with the organization of the different gene segments being broadly comparable to that of the B cell receptor.
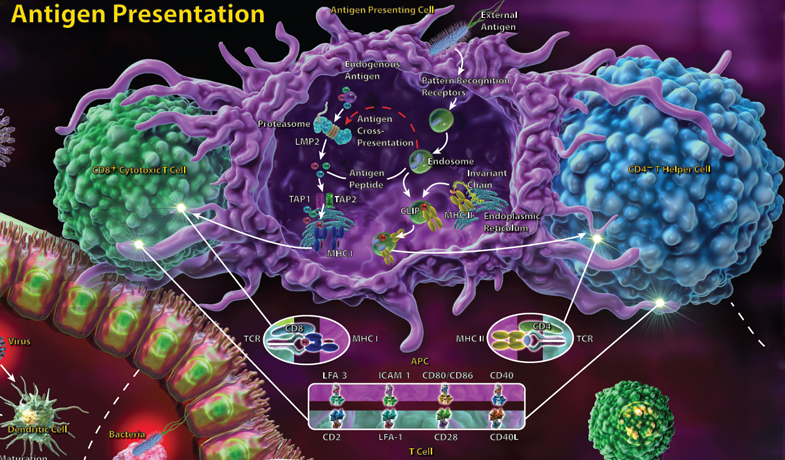
T cells recognize their antigen only when it is presented by MHC molecules, with CD4+ T helper cells recognizing antigen presented by MHC class II molecules and CD8+ cytotoxic T cells recognizing antigen presented in the context of MHC class I. The adaptive immune response involves CD4+ T helper cells, which, upon antigen recognition, receive many additional signals that determine which type of effector cell they will become. T helper cells may differentiate into Th1, Th2 or Th17 subsets, all of which have distinct roles.
 Login/Register
Login/Register 






Follow Us