- Clone
- GL3 (See other available formats)
- Regulatory Status
- RUO
- Other Names
- T cell receptor γ/δ
- Isotype
- Armenian Hamster IgG
- Ave. Rating
- Submit a Review
- Product Citations
- 40 publications
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 118107 | 25 µg | $67 | ||||
| 118108 | 100 µg | $144 | ||||
T cell receptor (TCR) is a heterodimer consisting of an α and a β chain (TCR α/β) or a γ and a δ chain (TCR γ/δ). TCR γ/δ belongs to the immunoglobulin superfamily, which is involved in the recognition of certain bacterial and tumor antigens bound to MHC class I. γ/δ TCR associates with CD3 and is expressed on a T cell subset found in the thymus, the intestinal epithelium, and the peripheral lymphoid tissues and peritoneum. Most γ/δ T cells are CD4-/CD8- although some are CD8+. T cells expressing the γ/δ TCR have been shown to play a role in oral tolerance, tumor-associated tolerance, and autoimmune disease. It has been reported that γ/δ T cells also play a principal role in antigen presentation.
Product DetailsProduct Details
- Verified Reactivity
- Mouse
- Antibody Type
- Monoclonal
- Host Species
- Armenian Hamster
- Immunogen
- C57BL/6J intraepithelial lymphocytes
- Formulation
- Phosphate-buffered solution, pH 7.2, containing 0.09% sodium azide.
- Preparation
- The antibody was purified by affinity chromatography, and conjugated with PE under optimal conditions.
- Concentration
- 0.2 mg/ml
- Storage & Handling
- The antibody solution should be stored undiluted between 2°C and 8°C, and protected from prolonged exposure to light. Do not freeze.
- Application
-
FC - Quality tested
SB - Reported in the literature, not verified in house
- Recommended Usage
-
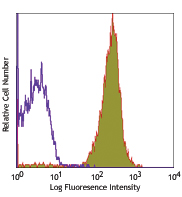
Each lot of this antibody is quality control tested by immunofluorescent staining with flow cytometric analysis. For flow cytometric staining, the suggested use of this reagent is ≤ 0.25 µg per 106 cells in 100 µl volume. It is recommended that the reagent be titrated for optimal performance for each application.
- Excitation Laser
-
Blue Laser (488 nm)
Green Laser (532 nm)/Yellow-Green Laser (561 nm)
- Application Notes
-
The GL3 antibody has been shown to be useful in identifying γ/δ T cells by flow cytometry and immunohistochemistry and depleting γ/δ T cells in vivo. Additional reported applications (for the relevant formats) include: immunoprecipitation1, immunohistochemistry of acetone-fixed frozen sections2,6, and in vivo depletion of γ/δ T cells3-5.
- Additional Product Notes
-
Iterative Bleaching Extended multi-pleXity (IBEX) is a fluorescent imaging technique capable of highly-multiplexed spatial analysis. The method relies on cyclical bleaching of panels of fluorescent antibodies in order to image and analyze many markers over multiple cycles of staining, imaging, and, bleaching. It is a community-developed open-access method developed by the Center for Advanced Tissue Imaging (CAT-I) in the National Institute of Allergy and Infectious Diseases (NIAID, NIH).
-
Application References
(PubMed link indicates BioLegend citation) -
- Goodman T, et al. 1989. J. Exp. Med. 170:1569. (FC, IP)
- Cardona AE, et al. 2003. Infect. Immun. 71:2634. (IHC)
- Kapp JA, et al. 2004. Immunology 111:155. (Deplete)
- Skelsey ME, et al. 2001. J. Immunol. 166:4327. (Deplete)
- Ke Y, et al. 1997. J. Immunol. 158:3610. (Deplete)
- Podd BS, et al. 2006. J. Immunol. 176:6532. (IHC)
- Kasten KR, et al. 2010. Infect. Immun. 78:4714. (FC) PubMed
- Stadanlick JE, et al. 2011. J. Immunol. 187:664. PubMed
- Van Belle AB, et al. 2012. J. Immunol. 188:462. PubMed
- Product Citations
-
- RRID
-
AB_313831 (BioLegend Cat. No. 118107)
AB_313831 (BioLegend Cat. No. 118108)
Antigen Details
- Structure
- Ig superfamily, associates with CD3 complex.
- Distribution
-
T cell subset in thymus, intestinal epithelium, peripheral lymphoid tissues and peritoneum, most γ/δ T cells are CD4-/CD8-, some are CD8+.
- Function
- Antigen recognition; γ/δ T cells are thought to play a role in tolerance.
- Ligand/Receptor
- Some bacterial or tumor antigens bound to MHC class I.
- Cell Type
- Epithelial cells, T cells, Tregs
- Biology Area
- Adaptive Immunity, Immunology
- Molecular Family
- TCRs
- Antigen References
-
- Skarstein K, et al. 1995. Immunology. 81:497.
- Harrison LC, et al. 1996. J Exp Med. 184:2167.
- Wildner G, et al. 1996. Eur J Immunol. 26:2140.
- Brandes M, et al. 2005. Science. 309:264.
- Gene ID
- 110066 View all products for this Gene ID 110067 View all products for this Gene ID
- UniProt
- View information about TCR gamma/delta on UniProt.org
Related FAQs
- What type of PE do you use in your conjugates?
- We use R-PE in our conjugates.
- If an antibody clone has been previously successfully used in IBEX in one fluorescent format, will other antibody formats work as well?
-
It’s likely that other fluorophore conjugates to the same antibody clone will also be compatible with IBEX using the same sample fixation procedure. Ultimately a directly conjugated antibody’s utility in fluorescent imaging and IBEX may be specific to the sample and microscope being used in the experiment. Some antibody clone conjugates may perform better than others due to performance differences in non-specific binding, fluorophore brightness, and other biochemical properties unique to that conjugate.
- Will antibodies my lab is already using for fluorescent or chromogenic IHC work in IBEX?
-
Fundamentally, IBEX as a technique that works much in the same way as single antibody panels or single marker IF/IHC. If you’re already successfully using an antibody clone on a sample of interest, it is likely that clone will have utility in IBEX. It is expected some optimization and testing of different antibody fluorophore conjugates will be required to find a suitable format; however, legacy microscopy techniques like chromogenic IHC on fixed or frozen tissue is an excellent place to start looking for useful antibodies.
- Are other fluorophores compatible with IBEX?
-
Over 18 fluorescent formats have been screened for use in IBEX, however, it is likely that other fluorophores are able to be rapidly bleached in IBEX. If a fluorophore format is already suitable for your imaging platform it can be tested for compatibility in IBEX.
- The same antibody works in one tissue type but not another. What is happening?
-
Differences in tissue properties may impact both the ability of an antibody to bind its target specifically and impact the ability of a specific fluorophore conjugate to overcome the background fluorescent signal in a given tissue. Secondary stains, as well as testing multiple fluorescent conjugates of the same clone, may help to troubleshoot challenging targets or tissues. Using a reference control tissue may also give confidence in the specificity of your staining.
- How can I be sure the staining I’m seeing in my tissue is real?
-
In general, best practices for validating an antibody in traditional chromogenic or fluorescent IHC are applicable to IBEX. Please reference the Nature Methods review on antibody based multiplexed imaging for resources on validating antibodies for IBEX.
Other Formats
View All TCR γ/δ Reagents Request Custom ConjugationCustomers Also Purchased
Compare Data Across All Formats
This data display is provided for general comparisons between formats.
Your actual data may vary due to variations in samples, target cells, instruments and their settings, staining conditions, and other factors.
If you need assistance with selecting the best format contact our expert technical support team.
-
Brilliant Violet 421™ anti-mouse TCR γ/δ
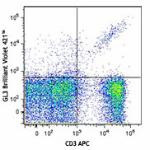

-
Purified anti-mouse TCR γ/δ
-
Biotin anti-mouse TCR γ/δ
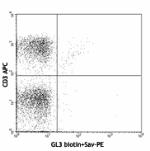
C57BL/6 lymph node cells stained with biotinylated GL3 and C... -
FITC anti-mouse TCR γ/δ
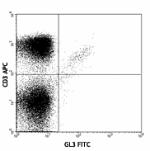
C57BL/6 lymph node cells stained with GL3 FITC and CD3 (145-... -
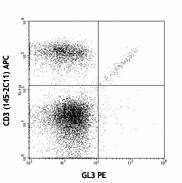
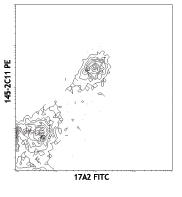
PE anti-mouse TCR γ/δ
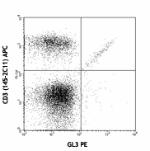
C57BL/6 splenocytes stained with CD3 (145-2C11) APC and GL3 ... 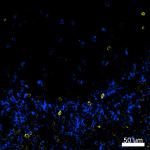
Confocal image of C57BL/6 mouse thymus sample acquired using... -
APC Anti-mouse TCR γ/δ
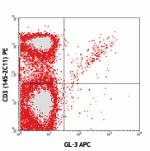
C57BL/6 mouse lymph node cells stained with CD3 (145-2C11) P... 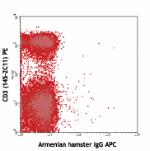
C57BL/6 mouse lymph node cells stained with CD3 (145-2C11) P... -
PerCP/Cyanine5.5 anti-mouse TCR γ/δ

C57BL/6 mouse splenocytes were stained with anti-mouse CD3ε ... -
PE/Cyanine7 anti-mouse TCR γ/δ

C57BL/6 mouse splenocytes were stained with CD3 APC and TCR ... -
Alexa Fluor® 488 anti-mouse TCR γ/δ
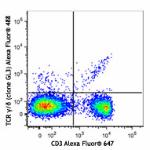
C57BL/6 mouse splenocytes were stained with CD3 Alexa Fluor®... 
-
Brilliant Violet 605™ anti-mouse TCR γ/δ
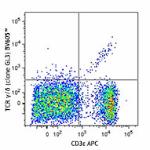
C57BL/6 mouse splenocytes were stained with CD3ε APC and TC... -
Brilliant Violet 510™ anti-mouse TCR γ/δ
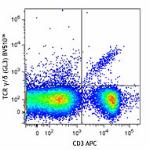
C57BL/6 mouse splenocytes were stained with CD3 APC and TCR ... 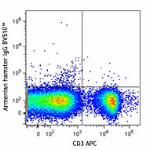
-
Alexa Fluor® 647 anti-mouse TCR γ/δ
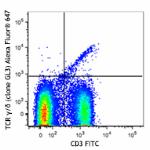
C57BL/6 mouse splenocytes were stained with CD3 FITC and TCR... 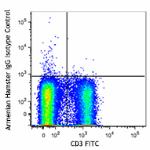
-
APC/Fire™ 750 anti-mouse TCR γ/δ
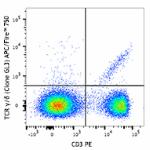
C57BL/6 mouse splenocytes were stained with CD3 PE and and T... 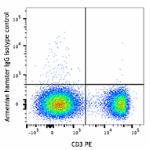
-
TotalSeq™-A0121 anti-mouse TCR γ/δ
-
Ultra-LEAF™ Purified anti-mouse TCR γ/δ
-
TotalSeq™-C0121 anti-mouse TCR γ/δ
-
APC/Cyanine7 anti-mouse TCR γ/δ
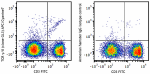
C57BL/6 mouse splenocytes were stained with anti-mouse CD3 F... -
TotalSeq™-B0121 anti-mouse TCR γ/δ
-
Brilliant Violet 650™ anti-mouse TCR γ/δ

C57BL/6 mouse splenocytes were surface stained with anti-mou... -
Brilliant Violet 711™ anti-mouse TCR γ/δ

C57BL/6 splenocytes were stained with anti-mouse CD3 FITC an... -
Spark Red™ 718 anti-mouse TCR γ/δ (Flexi-Fluor™)
-
Spark Blue™ 574 anti-mouse TCR γ/δ (Flexi-Fluor™)
-
Spark Blue™ 550 anti-mouse TCR γ/δ (Flexi-Fluor™)


 Login/Register
Login/Register 



















Follow Us