- Regulatory Status
- RUO
- Other Names
- Macrophage metalloelastase (MME), Matrix metalloproteinase 12
- Ave. Rating
- Submit a Review
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 768102 | 10 µg | $194 | ||||
| 768104 | 25 µg | $370 | ||||
Acetylcholinesterase (ACHE) is a member of the alpha/beta hydro lase superfamily. It is a serine hydrolase with a carboxyl esterase activity. Its main function is to terminate transmission at cholinergic synapses by hydrolyzing the neurotransmitter acetylcholine (ACH). Its inhibitors, such as donepezil, rivastigmine, and galantamine, have been approved to treat mild-to-moderate Alzheimer's disease and other dementia. Although ACHE is an essential enzyme primarily expressed in the central and peripheral nervous systems, its expression has been observed in various cell types including fibroblasts, osteoblasts, erythrocytes, vascular endothelial cells, and leukocytes. In addition to the main function, its role includes neurogenesis, neurodegeneration, apoptotic sensitivity, cellular proliferation and differentiation, and a possible role on tumorigenesis. There are three distinct alternative splicing isoforms, which mainly are different in C-terminal region after Thr574. Tetrameric ACHE, synaptic isoform (ACHE-S), expresses in major at brain and muscle tissue. Dimeric ACHE, erythrocytic isoform (ACHE-E), with modification of glycosylphosphatidylinositol (GPI) anchor, is an abundant form in human erythrocytes. The third readthrough isoform (ACHE-R) is a hydrophilic monomer produced at the cholinergic synapse in response to stress.
Product DetailsProduct Details
- Source
- Human ACHE, amino acids Arg34-Thr574 (Accession # NM_000665) with a C-terminal TG-8H-GGQ tag was expressed in CHO cells.
- Molecular Mass
- The 553 amino acid recombinant protein has a predicted molecular mass of approximately 60.7 kD. The non-reduced and DTT-reduced proteins migrate at 60 - 70 kDa by SDS-PAGE.
- Purity
- > 95%, as determined by Coomasie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in PBS at pH 7.2.
- Endotoxin Level
- Less than 1.0 EU per µg of protein as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for one month, at -20°C for three months, or at -70°C for six months. For maximum results, quick spin vial prior to opening. Avoid repeated freeze/thaw cycles.
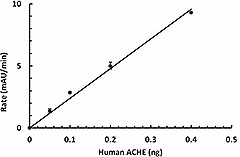
- Activity
- Human ACHE cleaves a chromogenic substrate acetylthiocholine with a specific activity value > 550 nmol/µg/min.
- Application
-
Bioassay
- Application Notes
-
Human ACHE Activity Assay
Human ACHE activity is measured by its ability to cleave the acetylthiocholine. The accumulation of the product cleavage is monitored by increase in intensity of absorbance at 405 nm in the presence of DTNB.
Materials and Buffers
- Human ACHE (Predicted MW: 60.7 kDa)
- Assay Buffer: 0.1 M sodium phosphate, 0.05% Brij-35, pH 7.5.
- 5’,5’-Dithiobis(2-nitrobenzoic acid) (DTNB)
- ACHE Substrate: Acetylthiocholine (ATC)
Assay Procedures
- Dilute rhACHE to 0.002 µg/mL.
- Dilute substrate to 200 µM in assay buffer containing 200 µM DTNB.
- Load 50 µL of rhACHE solution to clear plate. Include a control containing assay buffer only.
- Start reaction by adding 50 µL substrate/DTNB mixture.
- Read plate in kinetic mode for 5 minutes by monitoring absorbance at 405 nm.
- Calculate specific activity. Conversion factor of the product 5-thio-2-nitrobenzoic acid by using extinction coefficient 13260 M-1cm-1, 0.32 cm of light path correction, and total volume of 100 µL: 23.6 pmol/mAu
- rhACHE: 0.0001 µg
- DTNB: 100 µM
- ATC: 100 µM
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Monomer form
- Distribution
-
Muscle and brain, U2-OS, CACO-2, and Karpas-707 cell line.
- Function
- Cholinergic synapse transmission
- Interaction
- Acetylcholine
- Ligand/Receptor
- PRIMA1, COLQ
- Bioactivity
- Hydrolysis of acetylcholine
- Biology Area
- Neuroscience, Synaptic Biology
- Molecular Family
- Enzymes and Regulators
- Antigen References
-
1. Kandiah N, et al. 2017. Clin. Interv. Aging. 12:697.
2. Fujii T, et al. 2017. J. Pharmacol. Sci. 134(1):1-21.
3. Xi HJ, et al. 2015. Thorac. Cancer 6:390.
4. Zimmermann M, et al. 2008. J. Neurochem. 104:221.
5. Gorfe AA, et al. 2008. Biophys. J. 94:1144. - Gene ID
- 43 View all products for this Gene ID
- UniProt
- View information about ACHE on UniProt.org
Related Pages & Pathways
Pages
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)

 Login/Register
Login/Register 












Follow Us