- Regulatory Status
- RUO
- Other Names
- Fibroblast growth factor 1 (FGF-1), Acidic fibroblast growth factor (aFGF), Endothelial cell growth factor (ECGF), Heparin-binding growth factor 1 (HBGF-1)
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

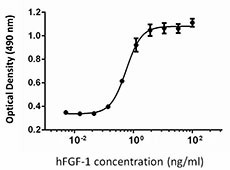
NIH3T3 proliferation induced by human FGF-1 in the presence of 10 µg/ml heparin.
FGF-1, one of the most studied members of the fibroblast growth factor family, is a powerful mitogen exhibiting strong action on many different cell types. FGF-1 activity can be mediated not only by autocrine/paracrine pathways but also by an intracrine pathway. FGF-1 lacks a secretion signal peptide and is exported through a non-classical pathway. Endogenous FGF-1 is found in the nucleus of most cell types. Nuclear localization is required for FGF-1 mitogenic activity. FGF-1 promotes tumor development by promoting cancer cell proliferation and survival. Increased FGF-1 expression in early stages of many different cancers has been reported. MCF-7 breast cancer cell line overexpressing FGF-1 can form vascularized, metastatic tumors when injected into ovariectomized or tamoxifen-treated nude mice. FGF-1 also induces angiogenesis in vitro and in vivo. Thus, FGF-1 is an attractive candidate for cancer immunotargeting. FGF-1 is also involved in neuronal cell differentiation and survival. FGF-1 is highly expressed in motor neurons. In response to damage, motor neurons can release FGF-1 which results in astrocyte activation. Under oxidative stress, astrocytes can also release FGF-1 which stimulates ApoE/HDL generation in an autocrine manner for protection of the brain against oxidative stress. Involvement of FGF-1 in inflammation, cardioprotection, wound healing, adipocyte remodeling, and restenosis is also reported.
Product DetailsProduct Details
- Source
- Human FGF-1, amino acids Phe16 - Asp155 (Accession# NP_000791) with an N-terminal Met, was expressed in E. coli.
- Molecular Mass
- The 140 amino acid recombinant protein has a predicted molecular mass of approximately 16 kD. The DTT-reduced and non-reduced protein migrates at approximately 17 kD by SDS-PAGE. The predicted N-terminal amino acid is Met.
- Purity
- >97%, as determined by Coomassie stained SDS-PAGE.
- Formulation
- 0.22 µm filtered protein solution is in 20 mM MOPS, 100 mM NaCl, pH 7.0.
- Endotoxin Level
- Less than 0.01 ng per µg cytokine as determined by the LAL method.
- Concentration
- 10 and 25 µg sizes are bottled at 200 µg/mL. 100 µg size and larger sizes are lot-specific and bottled at the concentration indicated on the vial. To obtain lot-specific concentration and expiration, please enter the lot number in our Certificate of Analysis online tool.
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week and stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- The ED50 is 0.2 - 1.0 ng/ml, corresponding to a specific activity 1.0 - 5.0 x 106 units/mg, as determined by a dose-dependent stimulation of NIH3T3 cell proliferation in the presence of 10 µg/ml heparin.
- Application
-
Bioassay
- Application Notes
-
This product is reactive with human, mouse, cow, pig, rat, shark, squid, hamster, hagfish, and leech.
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal or better stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
Antigen Details
- Structure
- Growth factor
- Distribution
-
FGF-1 is widely expressed in developing and adult tissues.
- Function
- FGF-1 plays important roles in many biological processes, including cell proliferation, inflammation, and angiogenesis. FGF-1 has low stability and a very short half-life in vivo. Binding to heparin increases the stability of FGF-1 and is also important for the formation of active FGF-acidic/FGFR complex.
- Interaction
- Intracellular FGF-1 can interact directly with CK2, FIBP, mortalin, and the ribosome-binding protein p34/leucine-rich repeat containing 59 (LRRC59).
- Ligand/Receptor
- FGF-1 can signal through all known cell-surface FGFR isoforms (FGFR1b, 1c, 2b, 2c, 3b, 3c, and 4). FGF-1 also binds to heparin and heparin sulfate proteoglycans on cell surface.
- Cell Type
- Embryonic Stem Cells, Hematopoietic stem and progenitors, Mesenchymal Stem Cells, Neural Stem Cells
- Biology Area
- Angiogenesis, Apoptosis/Tumor Suppressors/Cell Death, Cell Biology, Cell Cycle/DNA Replication, Cell Motility/Cytoskeleton/Structure, Immunology, Neuroscience, Stem Cells, Synaptic Biology
- Molecular Family
- Cytokines/Chemokines, Growth Factors
- Antigen References
-
1. Preta M, et al. 2005. Cytokine Growth Factor Rev. 16:159.
2. Zakrzewska M, et al. 2008. Crit. Rev. Clin. Lab. Sci. 45:91.
3. Zhang L, et al. 1997. Oncogene. 15:2093.
4. Lin YZ, et al. 1996. J. Biol. Chem. 271:5305.
5. Culajay JF, et al. 2000. Biochemistry. 39:7153.
6. Cassina P, et al. 2005. J. Neurochem. 93:38.
7. Pehar M, et al. 2005. Neurodegener. Dis. 2:139.
8. Jonker JW, et al. 2012. Nature. 485:391.
9. Compagni A, et al. 2000. Cancer Res. 60:7163. - Gene ID
- 2246 View all products for this Gene ID
- UniProt
- View information about FGF-1-acidic on UniProt.org
Related FAQs
- Why choose BioLegend recombinant proteins?
-
• Each lot of product is quality-tested for bioactivity as indicated on the data sheet.
• Greater than 95% Purity or higher, tested on every lot of product.
• 100% Satisfaction Guarantee for quality performance, stability, and consistency.
• Ready-to-use liquid format saves time and reduces challenges associated with reconstitution.
• Bulk and customization available. Contact us.
• Learn more about our Recombinant Proteins. - How does the activity of your recombinant proteins compare to competitors?
-
We quality control each and every lot of recombinant protein. Not only do we check its bioactivity, but we also compare it against other commercially available recombinant proteins. We make sure each recombinant protein’s activity is at least as good as or better than the competition’s. In order to provide you with the best possible product, we ensure that our testing process is rigorous and thorough. If you’re curious and eager to make the switch to BioLegend recombinants, contact your sales representative today!
- What is the specific activity or ED50 of my recombinant protein?
-
The specific activity range of the protein is indicated on the product datasheets. Because the exact activity values on a per unit basis can largely fluctuate depending on a number of factors, including the nature of the assay, cell density, age of cells/passage number, culture media used, and end user technique, the specific activity is best defined as a range and we guarantee the specific activity of all our lots will be within the range indicated on the datasheet. Please note this only applies to recombinants labeled for use in bioassays. ELISA standard recombinant proteins are not recommended for bioassay usage as they are not tested for these applications.
- Have your recombinants been tested for stability?
-
Our testing shows that the recombinant proteins are able to withstand room temperature for a week without losing activity. In addition the recombinant proteins were also found to withstand four cycles of freeze and thaw without losing activity.
- Does specific activity of a recombinant protein vary between lots?
-
Specific activity will vary for each lot and for the type of experiment that is done to validate it, but all passed lots will have activity within the established ED50 range for the product and we guarantee that our products will have lot-to-lot consistency. Please conduct an experiment-specific validation to find the optimal ED50 for your system.
- How do you convert activity as an ED50 in ng/ml to a specific activity in Units/mg?
-
Use formula Specific activity (Units/mg) = 10^6/ ED50 (ng/mL)

 Login/Register
Login/Register 






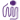





Follow Us